When new drugs enter the market, there are often delays before patients have access to the medication. These delays are partly due to patients and physicians being required to complete several steps before a prescription is approved for reimbursement. Alirocumab, a PCSK9 inhibitor which reduces low-density lipoprotein cholesterol (LDL-C)1, has up to a 75% rejection rate by payers2. The natural question that follows is what impact this delay may have on a patient’s health.
A poster presented at the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) modeled the impact on cardiovascular risk (CV) of delaying alirocumab treatment within a population with atherosclerotic cardiovascular disease (ASCVD)3. The analysis used anonymized patient-level data from the MarketScan Research Database and included patients with ASCVD, an LDL-C ≥ 70 mg/dL, and on a statin at baseline. Additional analysis focused on patients with genetically high cholesterol (heFH, heterozygous familial hypercholesterolemia) and patients with a recent acute coronary syndrome (ACS), such as a heart attack. As this was a real-world cohort, the baseline risks were estimated via Kaplan-Meier analyses for the cohort and relevant sub-populations. Risk reduction per unit reduction in LDL-C was based on CTT 20104. Additional assumptions are provided in the poster.
Download Report - "The Potential Of Real-World Evidence To Complement Basket Trials For Tissue"
The results showed the baseline CV risks of the cohort as well as the reduced risks with immediate treatment with alirocumab, alirocumab treatment after a 1-year delay, and alirocumab treatment after a 2-year delay. A 1-year delay caused an absolute increase of +1.5% to +3.7% on 3-year CV risks. A 2-year delay caused an absolute increase of +2.8% to +5.8% on 3-year risk.
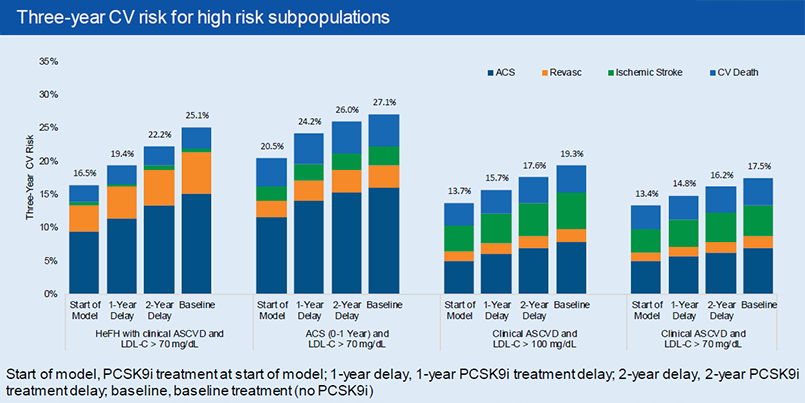
As was expected, a delay in alirocumab treatment yields higher risks than immediate alirocumab treatment, but lower risks than if alirocumab was never added to the statin therapy. What was interesting to note was both the magnitude of these increases as well the impact on different patient groups. Patients who have a higher baseline risk (heFH or recent ACS) have a greater absolute increase in risk due to treatment delays. Similarly, patients with a higher baseline LDL-C experience a greater absolute increase in risk due to treatment delays, holding all else equal.
Download Report - "Real-World Evidence Usage In Regulatory Approvals From USFDA And EMA"
It’s worth noting the impact on recent ACS patients, who are at highest risk of a subsequent CV event in that first year after their ACS event. Thus, they experience a greater increase in risk by delaying treatment in the first year than by delaying treatment in the second year.
This poster aimed to quantify the impact on risk of delaying treatment with a PCSK9 inhibitor. It further showed how the absolute increase in risk is most impactful for high-risk patients, patients with higher LDL-Cs, and patients with changing risks such as recent ACS patients. This information can be informative when evaluating the impact of a treatment delay on a particular patient.
Download Report - "Economic Burden And Modelling Techniques For Duchenne Muscular Dystrophy"
References
1. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm455883.htm.2. Baum SJ et al. Presented at the 66th Scientific Session of the American College of Cardiology, Washington, DC, USA. March 17-19, 2017.
3. https://www.ispor.org/research_pdfs/55/pdffiles/PCV64.pdf
4. Cholesterol Treatment Trialists’ Collaborators et al. Lancet 2012, 380:581-590.



