Medical Technology Guidance: A Review to Identify the Evidence Gaps and Data Needs
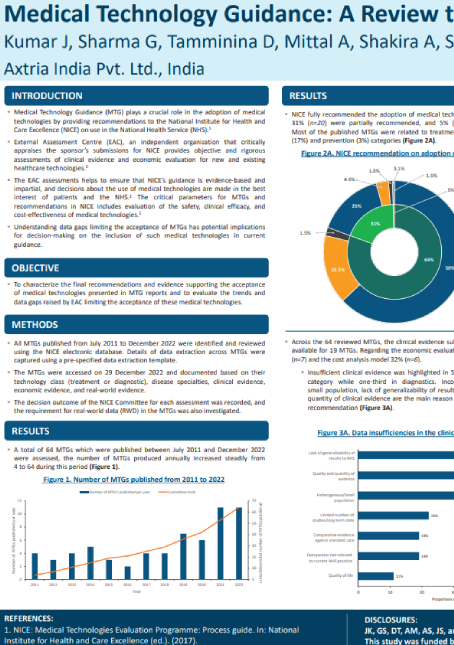
Medical Technology Guidance (MTG) plays a crucial role in the adoption of medical technologies by providing recommendations to the National Institute for Health and Care Excellence (NICE) on use in the National Health Service (NHS). The critical parameters for MTGs and recommendations in NICE includes evaluation of the safety, clinical efficacy, and cost-effectiveness of medical technologies. The purpose of this study is to characterize the final recommendations and evidence supporting the acceptance of medical technologies presented in MTG reports and to evaluate the trends and data gaps raised by EAC limiting the acceptance of these medical technologies.
Contact us at connect@axtria.com with any questions.
Recommended insights

Case Study

White Paper

Reports