Pharmaceutical companies heavily rely on key opinion leaders (KOLs) to create awareness about drugs and lend credibility to their products amongst the medical community. Hence, developing a KOL engagement strategy is critical for brand adoption.
Due to the significance of KOLs and the medical affairs programs that seek to engage them, it's crucial to accurately measure a KOL's relative influence and disease state expertise in an objective way. The breadth and depth of a KOL's involvement, brand adoption, and influence behaviors aids in deciding a KOL's strength and can be measured based on the following attributes:
- Disease State Expertise: KOLs may be named experts in a disease state, have authored papers, presented at forums, spoken in peer-to-peer events, or have been affiliated with key academic hospitals or disease state centers. Knowing a KOL – their research interests, contributions to the clinical and scientific literature, leadership contributions in medical societies, and associations – is vital before engagement activities begin.
- Sphere of Influence: KOLs may receive referrals or treat patients across geographical (national, regional, and local) and affiliation boundaries. This strategic view provides an advantage when designing subsequent KOL engagement and management processes.
- Concentration of Diagnosis/Treatment: A small number of healthcare professionals (HCPs) could be crucial to diagnosis, or treatment initiation, among a disproportionate number of patients. For example, in oncology, no one physician can be an expert across all the topics, tumor types, and biomarkers. Thus, community oncologists rely on experts who specialize in a specific tumor or state to inform them of new and better diagnostics and treatments.1
- Adverse Selection: KOLs may specialize in more-complex cases with higher risk, probability of disease relapse, and patients in later lines of therapy. For example, the diagnosis and treatment of a relapsed/refractory rare lung disease is a long journey for patients, with multiple physicians involved in patient care. However, within the disease community, there is a group of physicians considered by their peers to be thought leaders. Their opinions help to understand and validate current and emerging therapies for rare lung diseases.
- Early Adoption: KOLs may be early adopters of specific novel therapies and genetic testing types (e.g., cytogenetic testing). Early adopters are often involved in clinical trials and gain experience in a specific therapy area. They make an early assessment of the drug and are therefore important for the initial uptake of the drug and influencing trials broadly.
- Clinical Trial Participation: In some cases, a KOL may be a principal investigator (PI) for a clinical trial or possibly refer patients for participation in trials. The PI is responsible for coordinating the clinical trial process, which is particularly important when multiple study sites are involved. Therefore, the involvement of KOLs can be vital in ensuring the success of a clinical drug development program. In oncology, investigator-initiated trials are often conducted by thought leaders to experiment with ways to use a specific molecule in combination or for different tumors and indications, creating life-cycle extension opportunities.1
- Network Centrality: KOLs may receive many patients as inward referrals from a large number of HCPs.
KOL Identification and Mapping
The advent of data-driven technology is shaping the KOL identification and mapping process. KOL mapping is a quantitative approach to identifying important KOLs on a local, regional, and national level.2 There are several data sources and dimensions from which to gather information about KOLs. The more data collected, the smarter the decision-making should be. An effective approach for KOL identification and mapping involves integrating multiple secondary, public domain, and primary data sources to paint an influence network and identify the most influential physicians. These data sources include:
- Literature Review: Identifying key authors, researchers, and speakers based on publication and citation history (from sources such as PubMed®) and speaking roles, is useful to identify and profile KOLs of national significance. Further, pharmaceutical companies could also consider physicians in leadership roles in advisory boards and committees (such as the National Comprehensive Cancer Network, American Society of Clinical Oncology, American Heart Association, and American Rheumatism Association) for finding the most influential physicians.
- Longitudinal Patient Claims Data: This type of data provides information on patient populations or sub-populations treated, lines of therapies the KOLs are active in, and the regimens used, network centrality, and sphere of influence. Longitudinal patient claims data can be crucial for finding the most influential physicians in a specific therapeutic area. By collecting diagnosis and procedure level claims data, one can connect HCP profiles to their appropriate work with patients. This data can also be filtered by institutions and by region.
- Clinical Trials Data: Pharmaceutical companies can gather clinical trial data from the primary source, such as clinicaltrials.gov, or secondary datasets that build off the clinicaltrials.gov database, such as Trialtrove and Sitetrove. Data points such as the nature of the trial (interventional/observational), trial site, time period, trial status and outcome, primary investigator(s), and protocol/indication (e.g., relapsed/refractory and fit/unfit patient sub-populations) can help to identify a KOLs role in clinical trials. The publications associated with a trial, along with the authors, can also be identified. Other data points, such as who is funding the trial, can help get an overall view of all the parties engaged by a pharmaceutical company.
- Open Payments Data: This type of data helps identify potential speakers or influencers in peer-to-peer programs. However, integration of Open Payments data with other data sources requires understanding the nuances in the dataset, as illustrated below in Figure 1:
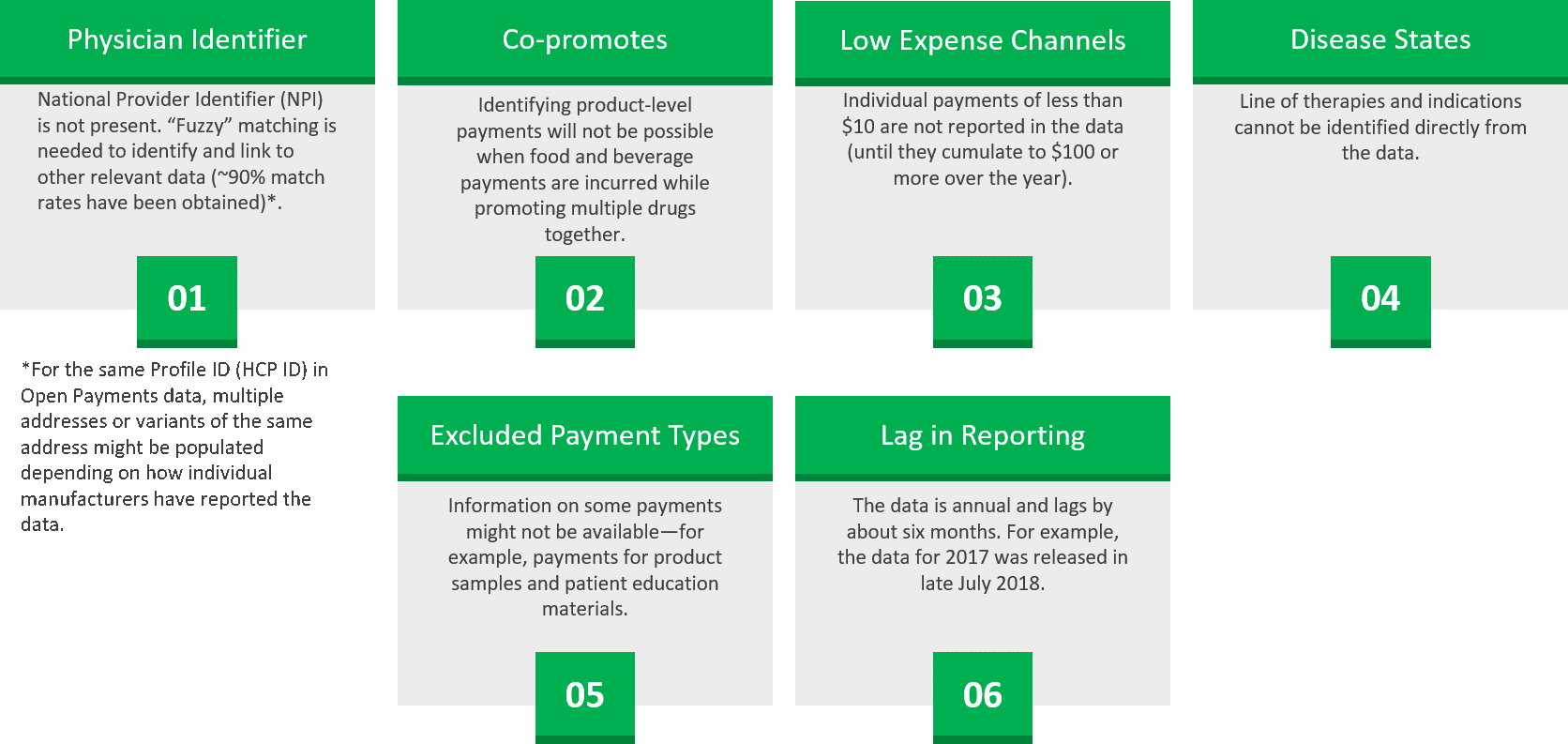
Figure 1 - Limitations of Open Payments Data
Source: Axtria, Inc.
- Social Media Data: Monitoring social media channels such as Twitter, YouTube, physician-only networks, and blogs can help identify HCPs who are digital opinion leaders (DOLs use the social media platform to offer opinions on new therapies, advise on disease and patient management, and discuss presentations from medical conferences and events). To identify DOLs, pharmaceutical companies need to look at quantitative (reach, frequency of postings, and resonance) and qualitative aspects (relevance of content and audience) of their online activity. While traditional KOLs (HCPs who share thought leadership by publishing research or speaking at conferences) might not have the same reach as DOLs on social media, some KOLs become DOLs over the years, and some may even start out as both.
- Focus Groups/Surveys: KOL data gathered through quantitative and qualitative research techniques can be valuable to identify known disease state authorities at national and regional levels.
- Affiliations Data: This data type can help identify a KOL's affiliation with known disease state centers and sphere of influence (e.g., receiving referrals within, versus across-integrated delivery networks and hospitals).
Further, KOL identification and mapping must involve combining all the right data sources with analytics to get the best results. Advanced analytics and visualization tools can be used to examine HCP data, identify thought leaders, and the strength of their connections. Each shortlisted HCP can be ranked on specific attributes based on a scoring system. The consolidated view across all attributes helps to arrive at a KOL/not-KOL decision. KOLs can further be classified as national, regional, or local–based on their sphere of influence. KOL profiles can be created, and relationships can be mapped with the help of visualization tools. Visualization tools (e.g., Neo4j is a graph database that facilitates relationship discovery and mapping to evolve customer profiles) help get a 360-degree view of KOLs and make it easier to visualize the strength of their connections.
Decisions Enabled through KOL Identification
The output is very granular and can be utilized by clients in numerous ways to impact product commercialization plans and tactics positively and allow a pharmaceutical company to more efficiently use its resources. Insights and downstream decisions enabled through KOL identification include:
- Tactical planning for KOL outreach and engagement
- Medical science liaison team sizing and deployment (based on number of KOLs and sphere of influence)
- Identification of speakers/peer-to-peer influencers
Conclusion
KOL identification and mapping involves analyzing several data sources for pharmaceutical companies to determine each KOL's level of influence to a very refined point. It offers the benefit of integrating multiple factors simultaneously into the identification process, providing the ability to target KOLs who offer well-rounded experience and expertise in a therapeutic area and who are well-connected. Engaging the right KOL can create much buzz around innovative products early on and help improve treatment outcomes.
However, with the increase in data available for analysis and the number of different ways to collect the information, there is no one-size-fits-all approach to KOL identification and mapping. Comprehensive and quality assured data sources should underpin any selection process. Also, the data is not useful unless appropriate filters and analytics are applied to provide actionable insights.
Ultimately, effective KOL identification and mapping can serve many purposes. Initiating the process with the right input from the outset of the project can help ensure its value to a pharmaceutical company.
Axtria has expertise across the KOL ecosystem, such as KOL identification, network visualization, and KOL profiling. Our analytics offer unparalleled insight into how experts rank professionally, how current their work is on a drug or indication, and who is in their professional network.
![]() Learn More - Developing Product Launch Strategy for an Orphan Drug For A Specialty Pharma
Learn More - Developing Product Launch Strategy for an Orphan Drug For A Specialty Pharma
References
- Myshko D. Oncology KOLS: An Important Piece of the Puzzle. September 2015. Available at: https://www.pharmavoice.com/article/2015-09-oncology-kol/
- Robinson R. KOL Mapping: The GPS of Thought Leader Identification. April 2011. Available at: https://www.pharmavoice.com/article/2011-04-kol-mapping/




